| 正极材料名称 |
化学式 |
合成方法 |
改性方法名称 |
具体改性方法 |
结构 |
形貌 |
结构与形貌图 |
放电容量 |
循环性能 |
倍率性能 |
热稳定性 |
改性效果图 |
备注 |
信息来源 |
收集人 |
校对人 |
| LiNi0.5Mn0.5O2 |
LiNi0.5Mn0.5-XAlXO2 |
共沉淀和后热处理法 |
掺杂Al改性 |
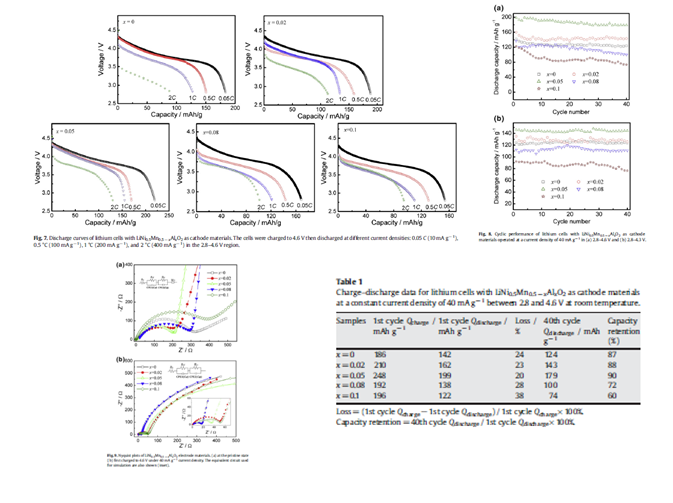
采用共沉淀和后热处理法制备了不同掺杂量的材料LiNi0.5Mn0.5?xAlxO2 (x=0, 0.02, 0.05,0.08, and 0.1) 。 |
无杂相,但晶格常数减少,结晶度提高 |
粒径增大 |

|
LiNi0. 5Mn0.45Al0.05O2: 199mAhg?1 (40mAg?1 in 2.8?4.6 V) |
40 循环后容量保持179mAhg?1 |
放电电流10, 100, 200, and 400 mAg?1 时,容量分别为219, 169, 155, and 129 mAhg?1 |
|
|
性能的改善是由于改善了材料的电子传递动力学 |
Bin Zhang、Gang Chen、Yilin Liang、Ping Xu. Structural and electrochemical properties of LiNi0.5Mn0.5?xAlxO2 (x=0, 0.02, 0.05,0.08, and 0.1) cathode materials for lithium-ion batteries. Solid State Ionics,2009,180:398?404. |
杨凯 |
倪海芳 |
| Li[Ni0.8Co0.15Al0.05]O2 |
Li[Ni0.8Co0.15Al0.05]O2 |
共沉淀法 |
包覆AlF3改性 |
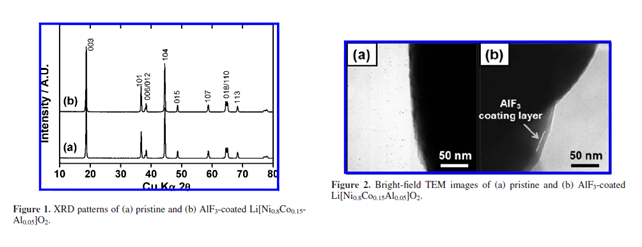
通过将Li[Ni0.8Co0.15Al0.05]O2先后与硝酸铝溶液和氟化铵溶液混合后干燥,在氧气气氛下400 ℃保温 5 h得到AlF3-Li[Ni0.8Co0.15Al0.05]O2 |
|
在颗粒表面均与包覆了大约10nm厚的AlF3包覆层 |
|
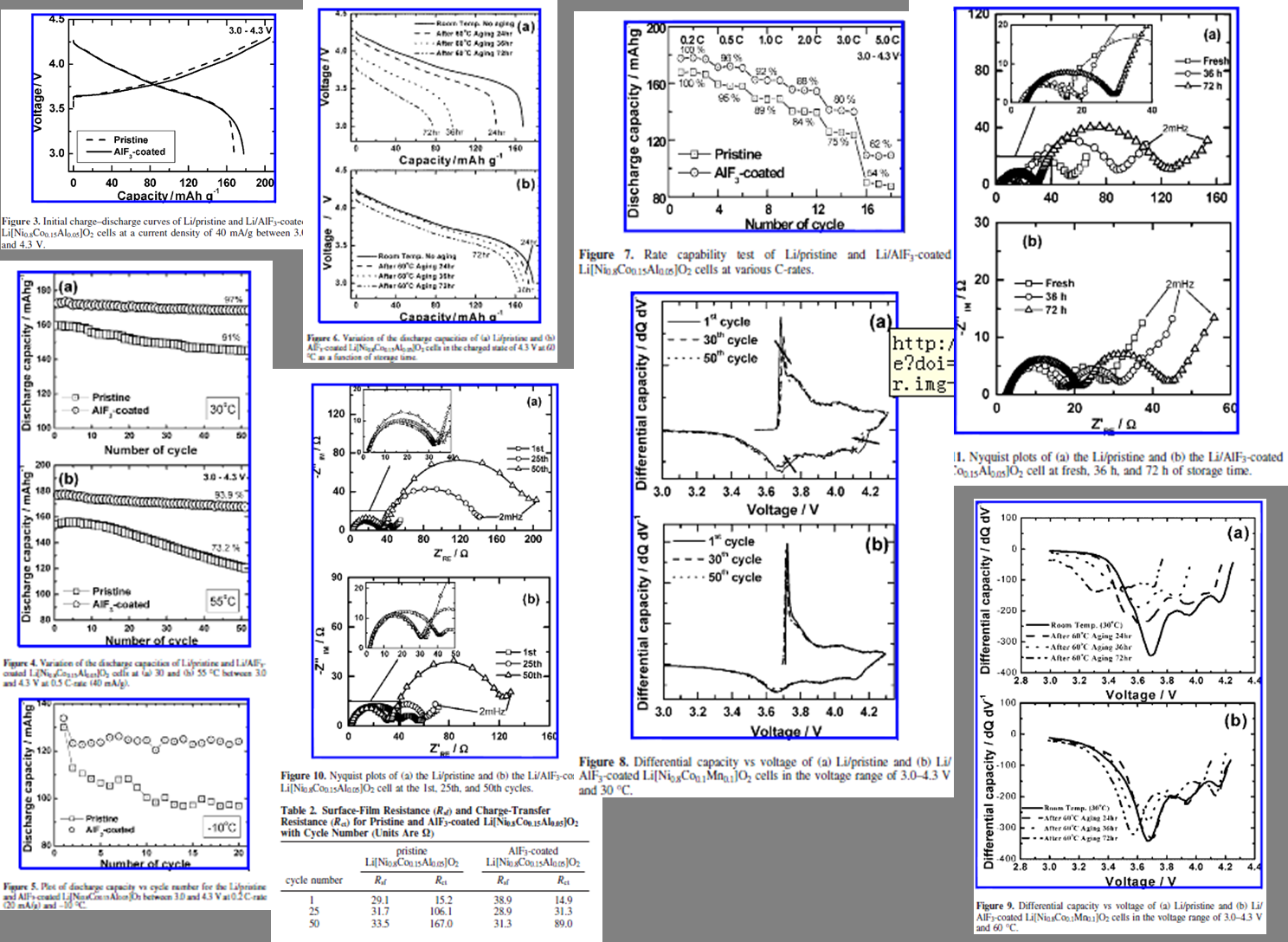
178mAh/g(40mA/g,3.0-4.3V),高于未改性的168 mAh/g。在60℃下储存72h能保留91%的容量,也高于未改性的46%。 |
在55℃下循环62次能保持94% 的容量,而为改性的样品只有 73% 。 |
1C和5C的放电容量分别还有92% 和 62% 高于为改性的89%和54% |
|
|
AlF3 在很好地抑制活性物质与电解质之间的副反应。 |
Byung-Chun Park、Hyung-Bae Kim、Hyun Joo Bang、Jai Prakash、Yang-Kook Sun. Improvement of Electrochemical Performance of Li[Ni0.8Co0.15Al0.05]O2 Cathode Materials by AlF3 coating at Various Temperatures. Ind. Eng. Chem. Res., 2008,47:3876?3882. |
杨凯 |
倪海芳 |
| 镍酸锂 |
LiNi0.9Co0.1O2 |
流变相法 |
Co掺杂改性 |
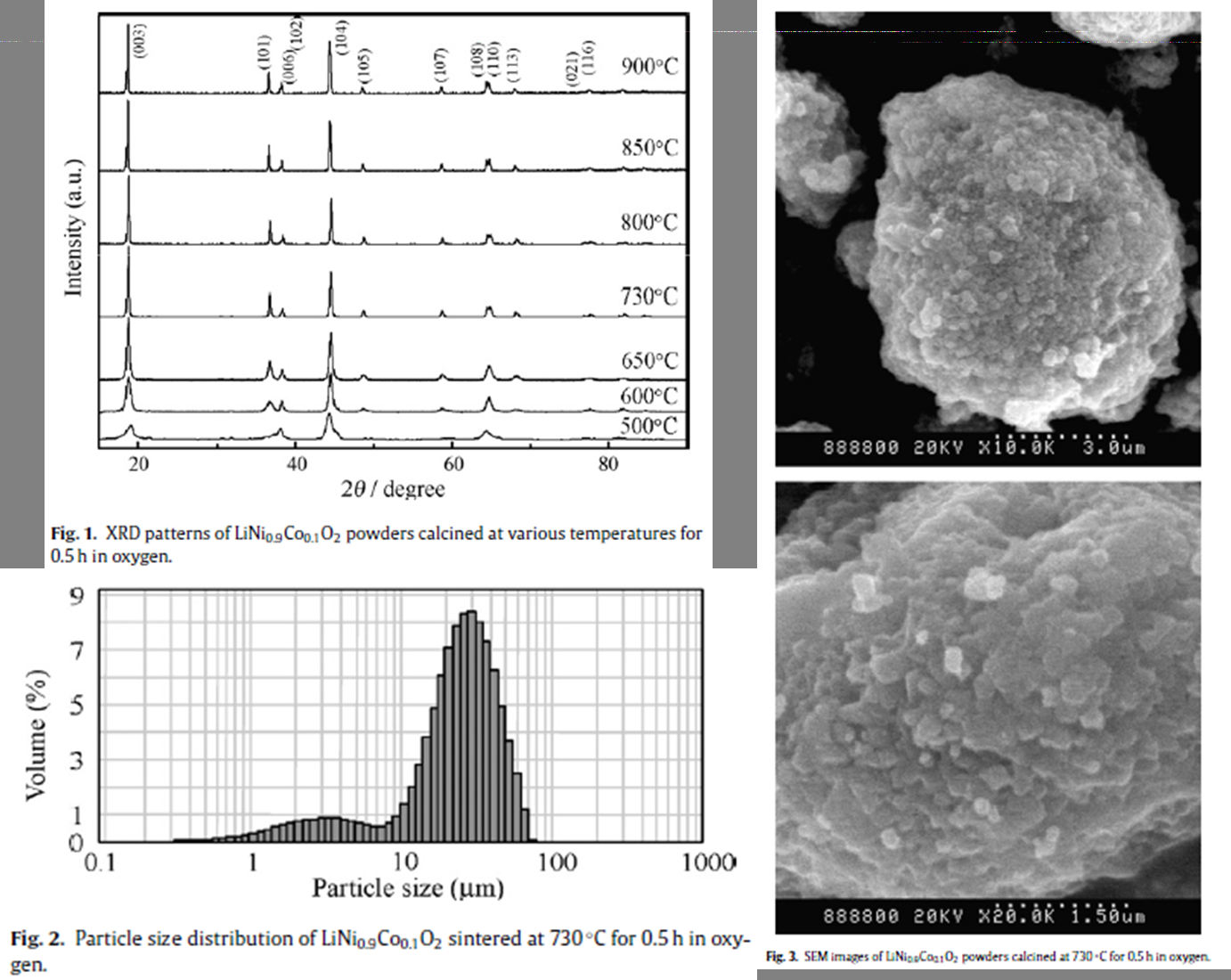
采用流变相法只煅烧0.5h就成功地合成出掺杂Co的LiNi0.9Co0.1O2 |
层状结构 |
平均粒径为25.299μm。 |
|
193mAhg?1(4.35-3.0V, 25mAg?1) |
15次循环后还有88.6% |
|
|

|
|
Xixi Shi、ChiweiWang、Xiaoling Ma、Jutang Suna. Synthesis and electrochemical properties of LiNi0.9Co0.1O2 cathode material forlithium secondary battery. Materials Chemistry and Physics,2009,113:780?783. |
杨凯 |
倪海芳 |
| 镍酸锂 |
LiNi0.9Al0.05Mg0.05O2 |
|
Al, Mg掺杂改性 |
|
单相层状结构 |
亚微米一次颗粒团聚形成平均粒径为5μm的球状二次颗粒 |

|
173mAhg?1 |
循环40次后容量还有少量增加 |
|
|

|
|
J. Kim、 B.H. Kim、Y.H. Baik、P.K. Chang、H.S. Park、K. Amine. Effect of (Al, Mg) substitution in LiNiO2 electrode for lithium batteries. Journal of Power Sources,2006,158:641?645. |
杨凯 |
倪海芳 |
| LiNi0.85-xCoxMn0.15O2 |
LiNi0.85-xCoxMn0.15O2 |
流变相法 |
Co掺杂改性 |
在氧气气氛下,采用流变相法在750℃保温10h制备出不同掺Co量的活性物质LiNi0.85-xCoxMn0.15O2 |
晶格常数减少 |
粒径为0.3μm的一次颗粒相互紧密团聚形成球状二次颗粒。 |

|
LiNi0.65Co0.20Mn0.15O2: 173.6mAhg?1 (50mA g?1, 3.0?4.3V) |
50次循环后还有90.6%的容量 |
|
放热反应温度274 ℃,高于LiCoO2的230℃ |

|
|
Daocong Li、Chaoqun Yuan、Jiaqing Dong、Zhenghe Peng、Yunhong Zhou. Synthesis and electrochemical properties of LiNi0.85-xCoxMn0.15O2 as cathode materials for lithium-ion batteries. J Solid State Electrochem, 2008,12:323?327. |
杨凯 |
倪海芳 |
| LiNi0.5Mn0.5O2 |
Li[Ni0.5Mn0.5]1-xCoxO2 |
超声喷雾热解法 |
Co掺杂改性 |
采用超声喷雾热解法制备不同掺杂量的球状形貌 Li[Ni0.5Mn0.5]1-xCoxO2 |
|
球状 |
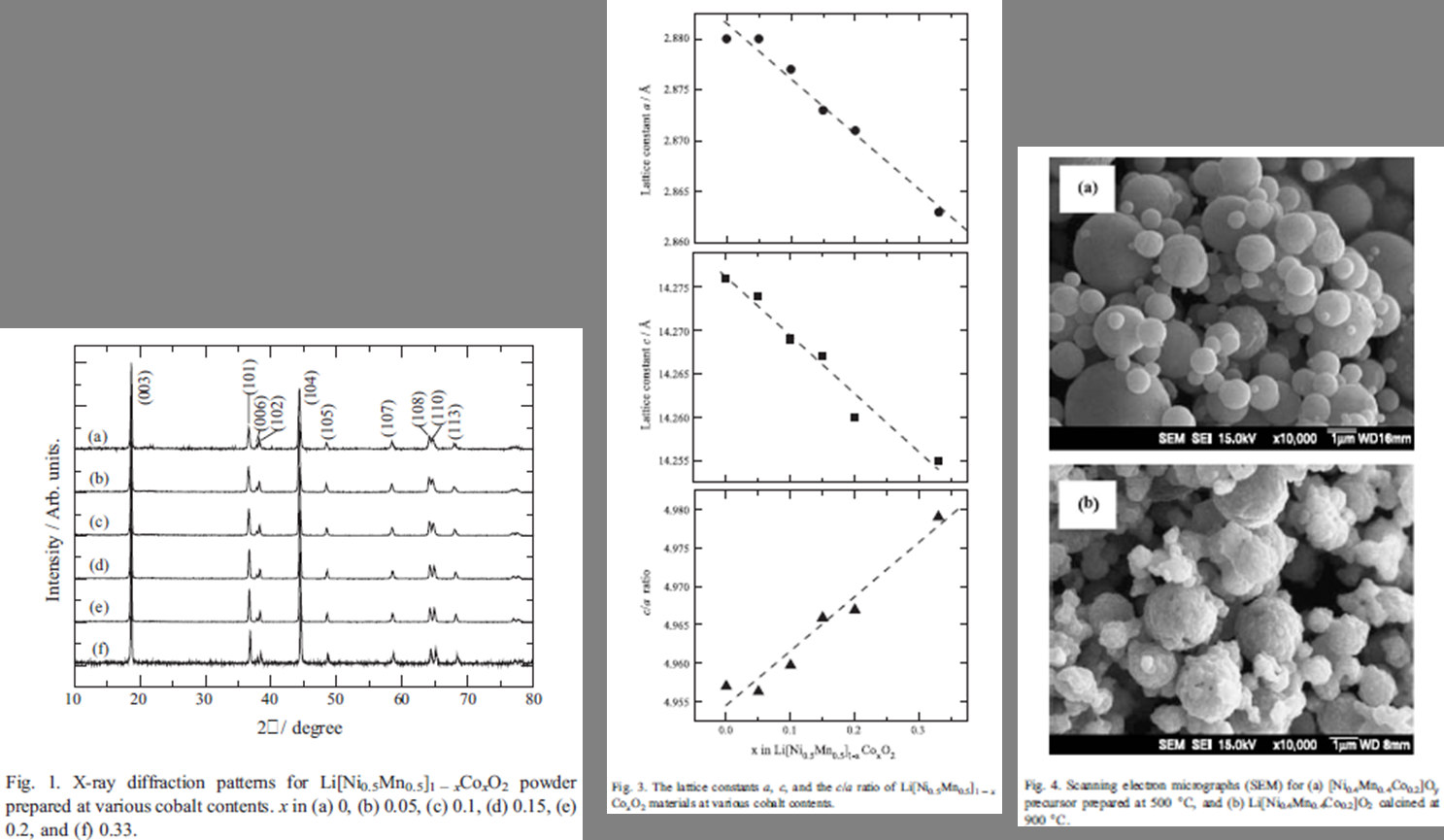
|
Li[Ni0.4Mn0.4Co0.2]O2 :2.8-4.4V, 175 mAhg-1 |
循环性能好 |
|
|

|
掺杂Co能有效提高材料的电导率,和结构稳定性。 |
S.W. Oh、SH Park、CW Park. Structural and electrochemical properties of layered Li[Ni0.5Mn0.5]1-xCoxO2 positive materials synthesized by ultrasonic spray pyrolysis method. Solid State Ionics,2004,171: 167?172. |
杨凯 |
倪海芳 |
| LiMn0.5Ni0.5O2 |
LiMn0.5?xCr2xNi0.5?xO2 |
化学溶液法 |
Cr掺杂改性 |
|
无杂相 |
|
|
当2x=0.05 大约145mAh g?1 (30 ℃,3?4.3V,5mAg?1) |
|
|
|
|
|
N.K. Karan、M. Balasubramanian、 D.P. Abraham、 M.M. Furczon、 D.K. Pradhan、J.J. Saavedra-Arias、 R. Thomas、 R.S. Katiyar. Structural characteristics and electrochemical performance of layered Li[Mn0.5?xCr2xNi0.5?x]O2 cathode materials, J. Power Sources,2009, 187:586?590. |
杨凯 |
倪海芳 |
| 镍酸锂 |
LiNi1-yTiyO2 |
高温固相法 |
Ti掺杂改性 |
|
随着掺杂量的增加,金属离子间的混排加剧。 |
|
|
掺杂使可逆比容量降低 |
|
|
|
|
|
L. Croguennec、 E. Suard、Willmann、 C. Delmas. Structural and Electrochemical Characterization of the LiNi1-yTiyO2 Electrode Materials Obtained by Direct Solid-State Reactions. Chem. Mater., 2002, 14: 2149-2157. |
杨凯 |
倪海芳 |
| 镍酸锂 |
LiNiO2 |
|
Ga 掺杂改性 |
将Ga盐与锂盐溶液混合干燥后在O2中煅烧 |
No impurity phase 无杂相,能掺杂能稳定其层状结构 |
|
|
190mAhg-1 (3.0-4.3 V) |
循环100次后容量保持率大于95%more than 95% after 100 cycles |
|
|

|
|
Yasunori Nishida、 Kenji Nakane、Tomoari Satoh. Synthesis and properties of gallium-doped LiNiO2 as the cathode material for lithium secondary batteries. Journal of Power Sources,1997, 68:561-564. |
杨凯 |
倪海芳 |
| 镍酸锂 |
LiNiO2 |
低温固相法 |
Mg掺杂改性 |
|
层状结构 |
均匀分布的粒径40nm的颗粒 |

|
190mAhg-1 (3.0?4.5V , 0.1mAcm?2 ) |
25循环后容量185.4mAhg-1 |
|
|

|
掺Mg减少晶格常数的变化,稳定结构,改善循环性能。. |
R. Sathiyamoorthi、 P. Shakkthivel、 S. Ramalakshmi、Yong-Gun Shul. Influence of Mg doping on the performance of LiNiO2 matrix ceramic nanoparticles in high-voltage lithium-ion cells. Journal of Power Sources,2007,171: 922?927. |
杨凯 |
倪海芳 |
| 镍酸锂 |
LiNiO3 |
溶胶凝胶法 |
Ga,Mg复合掺杂改性 |
|
六方层状结构,晶格常数增大 |
|
|
150 mAh/g (2.5?4.2 V, 0.2 mA/cm2) |
20次循环后稳定在150 mAh/g |
|
热稳定性得到改善 |

|
明显抑制了相转变 |
A. Yu、 G.V. Subba Rao、 B.V.R. Chowdari. Synthesis and properties of LiGaxMgyNi1-x-yO2as cathode material for lithium ion batteries. Solid State Ionics,2000,135:131?135. |
杨凯 |
倪海芳 |
| LixNi1-yCoyO2 |
LixNi1-yCoyO2 |
固相反应 |
MgO包覆改性 |
半结晶态的前躯体与醋酸镁混合煅烧得到MgO包覆的样品 |
|
|
|
首次放电容量降低 |
循环性能优异,1C下循环100次几乎不衰减 |
|
|
|
|
Ho-Jin Kweon、Sue Joo Kim、Dong Gon Park. Modification of LixNi1-yCoyO2 by applying a surface coating of MgO. Journal of Power Sources,2000,88:255?261. |
杨凯 |
倪海芳 |
| LiNi0.8Co0.2O2 |
LiNi0.78Co0.2Mg0.02O2 |
流变相法 |
Mg掺杂改性 |
Jiangfeng Xiang等人采用流变相法合成了掺杂Mg元素的LiNi0.8Co0.2O2。 |
层状结构 |
掺杂Mg后颗粒大小更均一 |
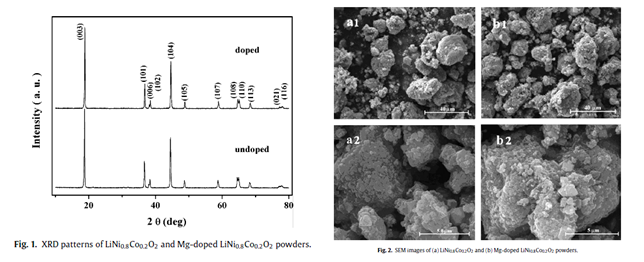
|
188mAhg?1(3.0-4.35V,0.5C) |
1C下50次循环后容量仅损失9%,而无改性的样品损失超过32% |
137mAhg?1 at 5C |
|

|
掺杂Mg抑制了材料充放电时电子传递和离子传递阻力的增加。 |
Jiangfeng Xiang、Caixian Chang、Feng Zhang、Jutang Sun. Effects of Mg doping on the electrochemical properties of LiNi0.8Co0.2O2 cathode material. Journal of Alloys and Compounds, 2009,475: 483?487. |
杨凯 |
倪海芳 |