| 正极材料名称 |
化学式 |
合成方法 |
改性方法名称 |
具体改性方法 |
结构 |
形貌 |
结构与形貌图 |
放电容量 |
循环性能 |
倍率性能 |
热稳定性 |
改性效果图 |
备注 |
信息来源 |
收集人 |
校对人 |
| 钴酸锂 |
LiCoO2 |
溶胶凝胶法 |
包覆改性 |
通过溶胶凝胶法在活性物质LiCoO2的表面上包覆一层Al2O3 |
|
表面上形成了一层纳米厚度固溶体LiCo1-yAlyO2 |

|
2.75-4.4V,0.5C,174mAhg-1 |
50次循环后保持94%,是未改性的两倍 |
|
|

|
颗粒表面形成的固溶体LiCo1-yAlyO2 为锂离子传输提供了快速通道,改善了材料的倍率性能;同时抑制了材料在充放电时的结构转变。 |
Jaephil Cho、Yong Jeong Kim、Byungwoo Park. Novel LiCoO2 Cathode Material with Al2O3 Coating for aLi Ion Cell. Chem. Mater., 2000, 12(12): 3788-3791. |
杨凯 |
雍颖琼 |
| 钴酸锂 |
LiCoO2 |
溶胶凝胶法 |
包覆改性 |
通过溶胶凝胶法在活性物质LiCoO2的表面上包覆一层TiO2 |
|
|
|
|
|
|
|
|
|
George Ting-Kuo Fey、Cheng-Zhang Lu、T.Prem Kumar、Yu-Chen Chang. TiO2 coating for long-cycling LiCoO2: A comparison of coating procedures. Surface & Coatings Technology, 2005, 199 (1): 22-31. |
杨凯 |
雍颖琼 |
| 钴酸锂 |
LiCoO2 |
溶胶凝胶法 |
金属氧化物(ZrO2、Al2O3、TiO2、B2O3)包覆改性 |
采用溶胶凝胶技术在材料钴酸锂表面包覆一层很薄的不同断裂韧性的金属氧化物。 |
包覆金属氧化物后能明显减少材料的应变,特别是包覆ZrO2后,材料几乎不发生应变。 |
|

|
|
70次循环后容量无衰减(2.75-4.4V,0.5C),而为改性的样品只有60% |
|
|

|
包覆明显抑制了材料的结构转变,因而改善了材料的循环性能。 |
Jaephil Cho、Yong Jeong Kim、Tae-Joon Kim、Byungwoo Park. Zero-Strain Intercalation Cathode for Rechargeable Li-Ion Cell. Angew. Chem., Int. Ed., 2001, 40(18): 3367-3369. |
杨凯 |
雍颖琼 |
| 钴酸锂 |
LiCoO2 |
|
AlPO4包覆改性 |
|
|
颗粒表面包覆了一层纳米尺度厚(大约3nm)的非晶状的AlPO4 |
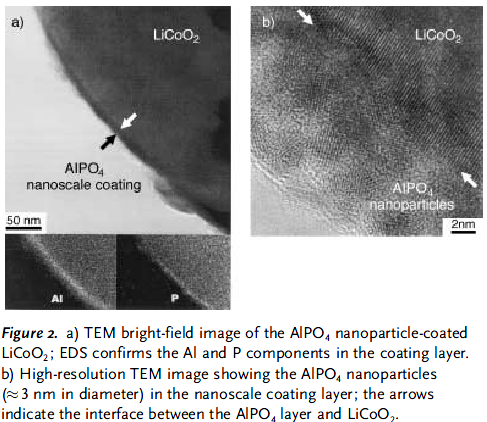
|
|
循环性得到明显的改善 |
|
明显改善了材料耐过充能力。当过充到12V时,电池内部最高温度只有60℃;而未经改性的样品达到500℃。 |

|
|
Jaephil Cho、Young-Woon Kim、Byoungsoo Kim、Joon-Gon Lee、Byungwoo Park. A Breakthrough in the Safety of Lithium Secondary Batteries by Coating the Cathode Material with AlPO4 Nanoparticles. Angew. Chem. Int. Ed., 2003, 42(14): 1618?1621. |
杨凯 |
雍颖琼 |
| 钴酸锂 |
LiCoO2 |
|
包覆改性 |
600℃保温加热5h分别在钴酸锂颗粒表面包覆一层P2O5和AlPO5 |
材料结构未改变 |
AlPO5包覆后,颗粒表面有一层厚度为10nm的AlPO5。 |

|
|
AlPO5包覆后循环稳定性得到改善(特别是在大电流下) |
|
包覆AlPO5和P2O5都能明显改善材料的热稳定性,提高材料的安全性。 |

|
|
Jaephil Cho、Joon-Gon Lee、Byoungsoo Kim、Byungwoo Park. Effect of P2O5 and AlPO4 Coating on LiCoO2 Cathode Material. Chem. Mater., 2003, 15(16): 3190-3193. |
杨凯 |
雍颖琼 |
| 钴酸锂 |
LiM0.05Co0.95O2(M=Bi、Sn、Cr、Zr) |
高温固相法 |
掺杂改性 |
采用高温固相法分别合成掺杂Bi、Sn、Cr、Zr的钴酸锂。 |
掺杂没有改变材料的衍射特征峰;除掺杂Bi外,材料没用出现别的杂峰,从衍射峰可以看出生成的材料层状特征非常明显。 |
|
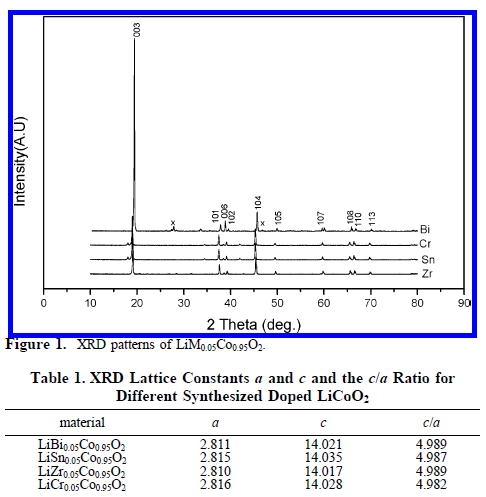
|
3.5-4.5V、0.2C,LiZr0.05Co0.95O2放电容量大约为155mAhg-1 |
循环性能非常好 |
|
|

|
材料性能改善的原因可能是Zr4+掺杂了部分Co3+,出现了部分Co2+,同时产生了一定的空位。 |
Meijing Zou、Masaki Yoshio、S. Gopukumar、Jun-ichi Yamaki. Performance of LiM0.05Co0.95O2 Cathode Materials in Lithium Rechargeable Cells When Cycled up to 4.5 V. Chem. Mater., 2005, 17(6): 1284-1286. |
杨凯 |
雍颖琼 |
| 钴酸锂 |
LiCo0.99Si0.01O2 |
共沉淀法 |
Si掺杂改性 |
采用共沉淀法能有效合成掺杂Si的钴酸锂。 |
掺杂量小于10%时,结构未发生变化 |
随着掺杂量的增加,颗粒变小。 |
|
|
|
|
|

|
阻抗降低,材料结构更稳定,循环性得到改善 |
Y. Jin、P. Lin、C.H. Chen. An investigation of silicon-doped LiCoO2 as cathode in lithium-ion secondary batteries. Solid State Ionics, 2006, 177: 317 ? 322. |
杨凯 |
雍颖琼 |
| 钴酸锂 |
LiCo1-xZrx/2Mgx/2O2 |
燃烧法 |
掺杂改性 |
采用燃烧法成功合成Zr和Mg复合掺杂的钴酸锂 |
|
|
|
|
|
|
|
|
|
H.Y. Xu、S. Xie、C.P. Zhang、C.H. Chen. Improving the electrochemical behavior of LiCoO2 electrode by mixed Zr?Mg doping. Journal of Power Sources, 2005, 148: 90?94. |
杨凯 |
雍颖琼 |
| 钴酸锂 |
|
溶胶凝胶法 |
金属氧化物包覆改性 |
|
|
|
|
|
70次循环后容量无衰减(2.75-4.4V,0.5C),而为改性的样品只有60% |
|
|

|
包覆处理后能显著抑制Co的溶解,提高材料的性能循环性能。 |
Yong Jeong Kim、Jaephil Cho、Tae-Joon Kim、 Byungwoo Parka. Suppression of Cobalt Dissolution from the LiCoO2 Cathodes with Various Metal-Oxide Coatings. Journal of The Electrochemical Society, 2003, 150(12): A1723-A1725. |
杨凯 |
雍颖琼 |